

Recent advancements in genetic testing have brought significant hope to families facing challenges related to fertility issues and genetic disorders. The Pioneers in Genetic Testing Innovative techniques in pre-implantation genetic testing (PGT) and non-invasive prenatal testing (NIPT) are
Artificial Intelligence has seen numerous applications in healthcare, yet one of the most promising areas is its ability to identify cognitive decline in menopausal women. Recent studies have indicated that machine learning can play a crucial role in recognizing women at risk of severe subjective
The transformative impact of rapid in-house next-generation sequencing (NGS) technology on cancer treatment is becoming increasingly evident. This advancement is particularly significant in enabling personalized medicine and improving patient outcomes. With the progress in molecular testing and the
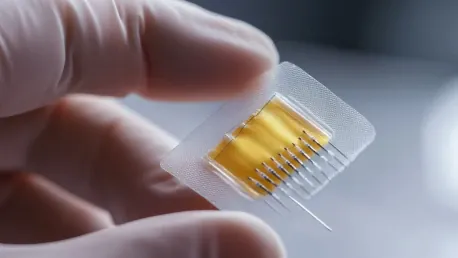
Imagine a world where getting vaccinated no longer means facing a needle and syringe, but instead, involves a simple patch applied to the skin. This prospect is rapidly becoming a reality thanks to the innovative work of the University of Queensland (UQ) and Brisbane-based biotech company Vaxxas.

Digital health venture funding in 2024 experienced notable shifts and trends that shaped the investment landscape. This article delves into the dynamics of venture capital investments, the trends in deal sizes and stages of funding, the influence of major investors, and the sectors within digital
A groundbreaking discovery in cancer research has unveiled a "genetic signature" involving 177 genes that are shared across multiple cancer types, fundamentally challenging prior assumptions about the mechanisms driving cancer metastasis. These findings open new avenues for innovative treatment
The advancements in muscle regeneration technology by Swiss biotech company MUVON Therapeutics are making waves, especially in the realm of treating stress urinary incontinence in women. This breakthrough holds the potential to improve the quality of life for millions while extending healthspan.

Amid growing discontent with the Turkish healthcare system, a coalition of health workers has taken decisive action to protest against new regulations they argue prioritize profit over patient care. Beginning with a five-day work stoppage on January 6, these protests culminated in a general

Stanford Medicine researchers have recently introduced an innovative artificial intelligence (AI) model named MUSK (multimodal transformer with unified mask modeling) that significantly improves the accuracy of cancer prognosis predictions. By integrating both visual and textual medical data, this
Geneoscopy, Inc., a life sciences company dedicated to advancing gastrointestinal health, has recently achieved an impressive milestone by securing a Series C funding round that amassed $105 million. This significant investment, led by Bio-Rad Laboratories and supported by various prominent
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy