

The realm of medical research often struggles with disentangling the intricate gene networks that underpin complex diseases, such as diabetes, cancer, and asthma. Single-gene disorders, such as cystic fibrosis or sickle cell anemia, can be traced to specific genetic mutations. However, the scenario
The synthetic biology market is on an unprecedented trajectory, projected to swell to $40 billion by 2032. This substantial growth reflects synthetic biology's rising influence across numerous critical sectors, including healthcare, agriculture, industrial biotechnology, and environmental

Imagine a future where cutting-edge medications reach patients in record time. This future is not a distant dream but a tangible possibility thanks to the FDA's introduction of fast-track vouchers under the Commissioner’s National Priority Voucher program. In an era demanding swift responses to

In the rapidly changing landscape of global security, the biodefense industry stands as a critical player, responding to increased threats of bioterrorism and infectious diseases. Governments worldwide are ramping up investments and developing policies to enhance emergency preparedness and
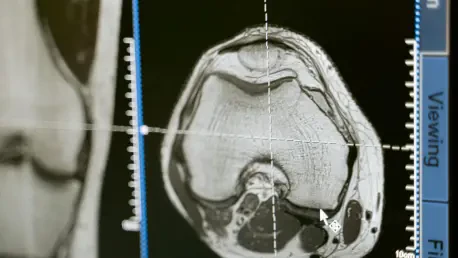
Could the future of brain tumor diagnosis truly be in the hands of artificial intelligence? As medical science rigorously explores the confluence of technology and healthcare, AI emerges as a groundbreaking tool in the world of cancer diagnostics. Specifically, this transformative technology

As temperatures cool and the common cold season descends, a significant health risk may be lurking unnoticed for many younger adults. Respiratory Syncytial Virus (RSV), once considered a pediatric and elderly threat, has raised concerns for adults aged 18 to 59 with underlying health conditions.

In recent years, the aging global population has brought significant attention to age-related health issues, especially sarcopenia—a condition marked by the loss of muscle mass and function. As traditional approaches such as exercise and nutrition struggle to counteract the cellular dysfunctions
Multiple myeloma remains a formidable challenge in oncology, known for its relentless cycle of relapse and treatment resistance. This blood cancer, which originates in plasma cells within the bone marrow, continues to affect patients profoundly, with around 6,240 individuals diagnosed annually in

The biopharmaceutical industry finds itself at a crossroads as the ever-increasing global demand for pharmaceuticals necessitates a reevaluation of traditional manufacturing approaches. With escalating costs and inefficiencies hindering timely drug production, the sector is turning towards a
The realm of genetic privacy has garnered profound attention from lawmakers in the United States as they confront unfolding challenges related to genetic information security and confidentiality. This year, legislative bodies have demonstrated a proactive stance on addressing pressing issues
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy