

Every year, countless men face a silent threat that often goes undetected until it’s too late—prostate cancer, which stands as one of the leading causes of cancer-related deaths among men globally. This disease claims lives with a stealth that makes early detection both a challenge and a necessity.

The landscape of clinical trials is undergoing a seismic shift as digital innovations reshape every aspect of research, with electronic consent (eConsent) emerging as a pivotal force in this transformation, especially as trials adopt decentralized and hybrid frameworks to accommodate diverse and

The spinner flask market is emerging as a cornerstone of laboratory equipment, particularly within the realm of cell culture technologies, as it addresses the growing needs of biotechnology and pharmaceutical sectors. These specialized vessels, engineered to cultivate cell cultures under

Imagine a scenario where a single mother in a rural community, struggling with diabetes, can access a dietitian’s expertise and receive medically tailored meals without ever stepping out of her home. This isn’t a distant dream but a tangible reality being shaped by the virtual foodcare movement, an
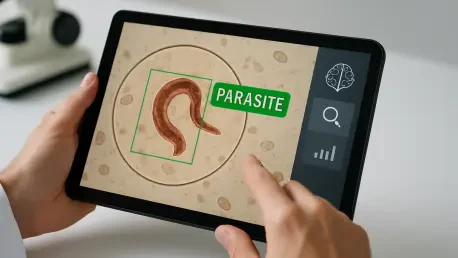
I'm thrilled to sit down with Ivan Kairatov, a biopharma expert with a wealth of experience in research and development, and a deep understanding of technological innovation in the industry. Today, we're diving into a groundbreaking advancement in clinical parasitology: an AI tool developed to
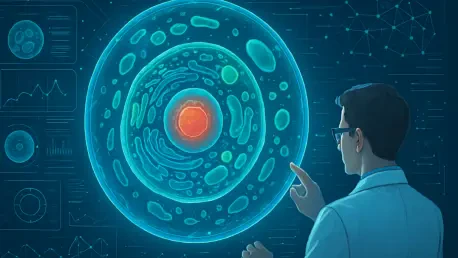
The realm of life sciences stands at a pivotal crossroads, grappling with an overwhelming deluge of biological data—petabytes of information that could unlock the secrets of human health but often remain untapped due to processing limitations. This challenge has spurred an innovative collaboration
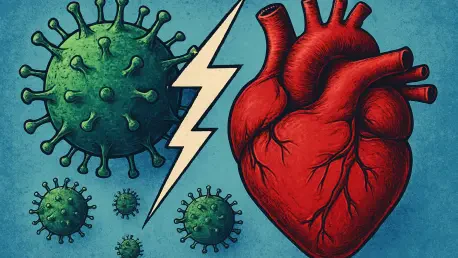
What happens when a seemingly harmless flu turns into a silent threat to your heart? Every year, millions battle common viruses like influenza or COVID-19, often shrugging them off as temporary inconveniences, yet beneath the surface, these infections may be laying the groundwork for something far

Every year, millions of lives are at stake due to delays in diagnosing severe heart attacks, particularly ST-segment elevation myocardial infarction (STEMI), a critical condition caused by a blocked coronary artery, and the difference between life and death often hinges on mere minutes. Timely

In the ever-evolving battle against cancer, a new frontier has emerged that promises to transform the landscape of treatment, particularly for stubborn solid tumors such as those found in breast, colorectal, pancreatic, and lung cancers. Natural Killer (NK) cell therapies, leveraging a unique

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in technology, innovation, and research and development within the industry. Today, we’re diving into the exciting world of biomanufacturing, focusing on a groundbreaking new lab at a prominent
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy