

In a landscape where patients with relapsed or refractory T-cell lymphoma face a grim prognosis, often with a median survival of just six months under current treatments, a new ray of hope has emerged through innovative research. This aggressive cancer, marked by limited therapeutic options, has
The landscape of rare disease drug development is littered with clinical failures. For every success, countless promising compounds fail to demonstrate efficacy in late-stage trials, often due to the inherent challenges of studying small, heterogeneous patient populations. BridgeBio Pharma’s recent

Imagine a patient facing major surgery, anxious about the risks and uncertain about recovery, only to discover that a tailored preparation plan could dramatically lower complications and improve outcomes. This isn’t a far-off dream but a reality backed by groundbreaking research from a leading

Imagine a world where cancer treatment is not just about managing a disease, but consistently achieving lasting remission through groundbreaking therapies that target cancer at its core. The oncology community stands on the brink of such transformative change, fueled by rapid advancements and an

In the high-stakes world of biopharmaceuticals, where developing a single drug can cost upwards of $2.7 billion, inefficiencies in clinical bioanalysis pose a significant threat to timelines and budgets, often delaying critical decisions by weeks or even months. Traditional manual methods,
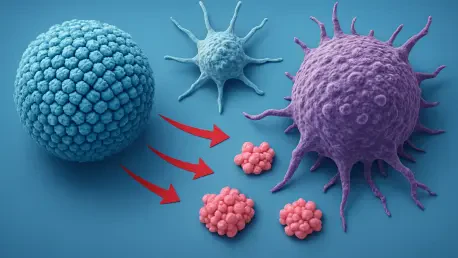
Diving into the forefront of cancer immunotherapy, we’re thrilled to speak with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development. Ivan’s deep knowledge of technological innovation in the industry has positioned him as a leading voice in the fight

In a remarkable stride toward redefining medical science, Mark Zuckerberg and Priscilla Chan, co-founders of Biohub, have launched an ambitious initiative that combines artificial intelligence (AI) with cutting-edge biology to revolutionize human disease research. This visionary project aims to

Across the globe, mental disorders stand as a formidable public health challenge, contributing to significant disability, premature mortality, and crushing economic burdens on both patients and healthcare systems. In China, where the lifetime prevalence of these conditions exceeds 16%, the

The COVID-19 pandemic has presented an unprecedented challenge to global health, revealing intricate and often devastating ways in which the SARS-CoV-2 virus disrupts the human body at a molecular level, particularly in severe cases characterized by rampant inflammation and life-threatening blood

In the ever-evolving landscape of cancer treatment, immunotherapies such as CAR T-cell therapy and T-cell engager therapies have emerged as revolutionary options, offering renewed hope to patients battling hematologic malignancies like lymphomas, leukemias, and multiple myeloma. However, these
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy