

Imagine a world where the mysteries of life at every scale—from the tiniest molecular interactions to the complex systems of entire organisms—are decoded with unprecedented speed and accuracy, potentially transforming how diseases are understood and treated. This vision is at the heart of a
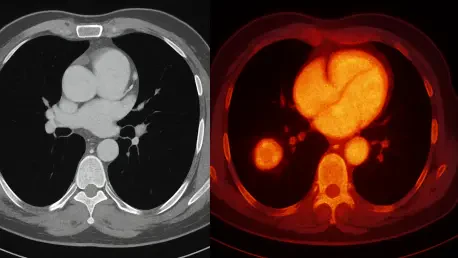
The landscape of medical diagnostics is undergoing a profound transformation with the emergence of an innovative dual-mode imaging system that seamlessly integrates X-ray and near-infrared (NIR) capabilities into a single, powerful scan. This cutting-edge technology, powered by bifunctional NIR
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in the intersection of technology and innovation in health sciences. Today, we’re diving into the fascinating world of the gut microbiome, exploring

In the complex landscape of women’s health, few conditions pose as significant a challenge as endometriosis and adenomyosis, disorders characterized by the abnormal growth of endometrial tissue outside the uterus or within its muscular wall. These conditions afflict millions of women globally,
What if a silent condition affecting millions could be stopped before it spirals into a life-altering crisis? Chronic kidney disease (CKD), impacting 8% to 16% of the global population, often progresses undetected until it reaches end-stage renal disease (ESRD), a point where kidneys function at
In a landscape where mesothelioma, a devastating cancer often linked to asbestos exposure, has long been synonymous with poor survival rates and limited treatment options, a new clinical trial offers a glimmer of hope that could redefine patient outcomes. Conducted by experts at the Johns Hopkins

Imagine a world where artificial intelligence (AI) in health care can diagnose diseases with pinpoint accuracy, yet for certain communities, these tools consistently deliver flawed results due to hidden biases in the data they rely on. This stark contrast between potential and reality underscores a
Imagine a world where chronic diseases like cardiovascular disorders, metabolic conditions, and autoimmune issues are treated not by altering the very fabric of DNA, but by subtly tweaking how genes are expressed—a safer, more precise approach to healing that could transform countless lives.

Cancer continues to be one of the most daunting challenges in modern healthcare, with millions of lives affected annually by its devastating impact, and traditional treatments like chemotherapy and radiation, while often effective, frequently bring debilitating side effects and inconsistent

In the rapidly advancing realm of cancer research, a transformative study from the University of East Anglia (UEA), recently published in Science Translational Medicine , has captured significant attention with its exploration of the microbial world within tumors. This research delves into whether
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy