

In a landscape where heart failure impacts over 6 million Americans and stands as a primary driver of hospitalizations and mortality, the urgency for innovative treatment solutions has never been greater, especially when traditional drug development often stretches over a decade and costs upwards

What if a simple device on your wrist could reveal the hidden shifts of pregnancy, potentially saving lives by detecting issues early? In a world where maternal health challenges persist, wearable sensors—commonly used for fitness tracking—are stepping into a groundbreaking role. These everyday
As the world navigates through 2025, the ongoing battle against cancer reveals a landscape of remarkable triumphs juxtaposed with daunting hurdles, as detailed in the American Association for Cancer Research (AACR) Cancer Progress Report released last year. This comprehensive document captures a
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, as well as a keen understanding of technological innovation in the industry. Today, we’re diving into the exciting world of precision oncology and drug discovery,

In a world where millions of families struggle with infertility, a groundbreaking advancement promises to reshape the landscape of reproductive medicine with innovative solutions. Conceivable Life Sciences, a pioneering company based in New York, has emerged as a beacon of hope with its latest

In an era where access to reliable health information can make a significant difference in public safety, South Korea's Ministry of Food and Drug Safety is taking bold steps to enhance its digital resources for medical devices. With millions of people relying on accurate data to make informed
Imagine a world where the agonizing wait for a life-saving organ transplant becomes a relic of the past, replaced by the ability to print a custom organ tailored to a patient’s unique biology, and Israel is stepping into this visionary future with the launch of a groundbreaking research institute
In the realm of oncology, bladder cancer remains a formidable adversary, claiming over 16,000 lives annually in the United States alone, with metastatic urothelial carcinoma (mUC) presenting particularly grim prospects. Imagine a scenario where a patient, battling advanced stages of this disease,

In an era where mental health care is increasingly vital, the administrative burdens on providers often hinder their ability to focus on patients, creating a significant gap between need and delivery that impacts the quality of care. A staggering number of clinicians spend hours on paperwork,
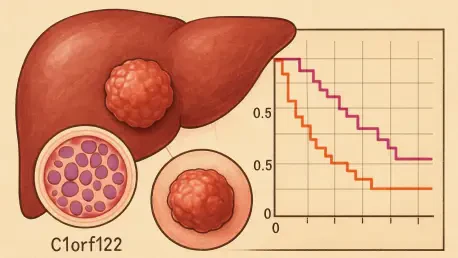
Imagine a disease so pervasive that it ranks as the third leading cause of cancer-related deaths worldwide, with a staggering 90% of primary liver cancer cases falling under its umbrella. Hepatocellular carcinoma (HCC) presents an immense challenge to global health systems, with rising incidence
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy