
Imagine a hidden cellular force driving a spectrum of diseases from acne to rheumatoid arthritis, silently shaping patient outcomes across the globe while costing healthcare systems billions annually. This is the reality of fibroblasts, specialized cells once thought to be mere structural

In the high-stakes arena of biopharmaceuticals, where the race to deliver life-saving therapies to patients is intensifying, a transformative collaboration between Abzena, Revvity, and ProteoNic Bioscience is making waves with significant upgrades to biomanufacturing processes. This partnership has
In a remarkable achievement that underscores a steadfast dedication to excellence in pediatric medical imaging, Children’s Hospital of Orange County (CHOC), part of Rady Children’s Health, has been named an inaugural recipient of the BeRAD Professionalism Award by the American Society of Radiologic
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert whose extensive experience in technology and innovation has shaped advancements in healthcare. With a strong background in research and development, Ivan brings a unique perspective on how cutting-edge tools like the new
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, particularly in innovative technologies shaping the future of healthcare. Today, we’re diving into a groundbreaking study from UCLA Health on the impact of regular

In the high-stakes environment of modern healthcare, computed tomography (CT) scanners stand as indispensable tools for diagnosing critical conditions with speed and precision, yet beneath their life-saving capabilities lies a troubling reality of excessive energy consumption. These machines often
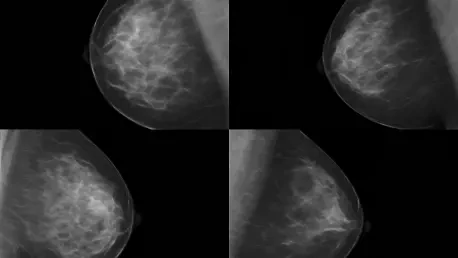
Today, we’re thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, and a deep understanding of technological innovation in the industry. Ivan has been closely involved in groundbreaking studies that are shaping the future of

In an era where technology has reshaped nearly every facet of daily life, access to virtual care through telehealth has emerged as a vital lifeline for millions of Americans seeking medical services without stepping foot in a clinic. The American Telemedicine Association (ATA), through its advocacy
What happens when a seemingly harmless growth turns into a silent, deadly threat with almost no viable treatments in sight, leaving patients and doctors desperate for solutions? Solitary fibrous tumors (SFTs), a rare type of soft tissue tumor, affect a small fraction of the population, yet for the

In the sprawling landscape of U.S. healthcare, a silent crisis unfolds every day as hundreds of thousands of patients suffer preventable harm or even death due to medical errors, positioning patient safety as an urgent public health challenge that demands immediate attention. With medical errors
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy