
In the realm of modern healthcare, ensuring the right drug dosage for each patient remains a formidable challenge, with studies revealing that up to 50% of patients on critical therapies like chemotherapy receive suboptimal doses due to individual variations in drug metabolism. This discrepancy
In the intricate world of cancer biology, the acidic environment within tumors, commonly known as acidosis, stands out as a formidable force that not only challenges cancer cells but also fuels their survival and growth, particularly in aggressive types like pancreatic cancer. Groundbreaking

The realm of cell and gene therapies (CGTs) has long been heralded as a revolutionary frontier in medicine, promising transformative outcomes for patients with previously untreatable conditions, yet it faces significant hurdles in securing consistent funding. As the industry matures, it confronts a

In an era where artificial intelligence is reshaping countless industries, a chilling possibility has emerged at the intersection of AI and synthetic biology, raising urgent questions about global safety. Imagine a scenario where malicious actors harness sophisticated algorithms to reengineer
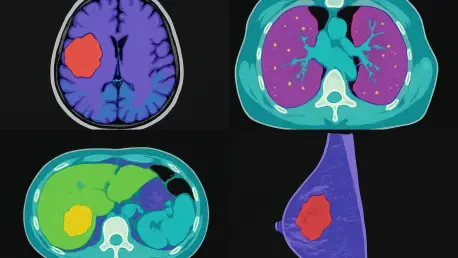
What if a technology could transform the way doctors analyze intricate medical scans, cutting down hours of work into mere minutes with unerring precision, while making diagnostics more accessible worldwide? Picture a hospital overwhelmed with brain MRI scans, where clinicians struggle to keep up

In a world where obesity rates have more than doubled since 1990, the accumulation of excess body fat, known as adiposity, has emerged as a critical public health challenge with profound implications for mortality and chronic disease risk. This staggering rise positions adiposity as a leading

Imagine a world where life-saving biologics are produced faster, more efficiently, and at a scale never seen before, directly impacting millions of lives across the globe with groundbreaking advancements. This vision is becoming reality in Europe, where the biotech sector is witnessing a

I'm thrilled to sit down with Ivan Kairatov, a seasoned biopharma expert with a wealth of experience in research and development, as well as a deep understanding of technological innovation in the industry. Today, we’re diving into the exciting collaboration between two leaders in laboratory
Imagine a scenario where a child undergoes surgery, and the anesthesiologist receives a critical alert about a potential oxygen drop 60 seconds before it becomes life-threatening, thanks to the power of artificial intelligence (AI) in pediatric anesthesia. This is no longer a distant dream but a

In a world where medical breakthroughs are happening at an unprecedented pace, the challenge of ensuring timely access to cutting-edge technologies while maintaining rigorous safety standards has never been more critical. A groundbreaking collaboration between the UK’s Medicines and Healthcare
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy