

The landscape of modern biotechnology is currently defined by a profound transition from the creation of isolated high-performance instruments to the development of fully unified experimental ecosystems. For many years, the pharmaceutical sector prioritized the acquisition of specialized tools that
The persistent erosion of communal structures in the digital age has transformed what was once a private sorrow into a measurable physiological threat that rivals traditional oncological risks. While medical science has long focused on genetic predispositions and environmental toxins, a silent
The architectural complexity of the tumor microenvironment reveals a sophisticated biological intelligence where immune cells, once thought to be defenders, are subverted into tactical accomplices for malignant growth. This transformation is driven largely by extracellular vesicles, which function

Ivan Kairatov is a distinguished biopharma expert with a career built on navigating the intricate crossroads of biotechnology innovation and regulatory strategy. With extensive experience in research and development, he has spent years analyzing how emerging therapies transition from the laboratory
The High Stakes of the Alzheimer’s Waiting Game A diagnosis of Alzheimer’s Disease often feels like being handed a map with the ink still wet, leaving families to navigate a landscape where the landmarks of memory and independence could vanish tomorrow or remain for a decade. This profound
The high-stakes world of oncology has long viewed triple-negative breast cancer as a fortress that resists the most sophisticated modern weapons, leaving patients and physicians with few options beyond traditional chemotherapy. This aggressive subtype, notorious for its rapid progression and lack

The ability to watch a single protein molecule decide the fate of a living cell has long been the holy grail of molecular biology, yet for decades, researchers remained trapped behind the blurry veil of static imaging. While traditional microscopy could tell us where a protein was located at a

The healthcare landscape across Southeast Asia is currently undergoing a radical transformation that prioritizes the unique biological identity of every patient over generalized treatment protocols. This shift is most visible in the emergence of dedicated precision medicine hubs, such as those
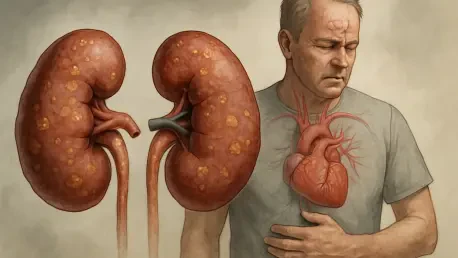
The silent progression of kidney disease has emerged as a formidable public health crisis in the United States, where nearly 15% of the adult population currently grapples with some form of renal impairment. These vital organs serve as the body's primary filtration system, meticulously removing

The silent progression of arterial damage and metabolic shift is quietly rewriting the biological future of nearly sixty percent of the female population in the United States. Recent scientific projections have cast a sobering light on the trajectory of women's heart health, suggesting that by the
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy