

Imagine a world where synthetic organisms, engineered with molecular structures completely alien to natural life, could proliferate unchecked, evading all known defenses and disrupting ecosystems on a global scale. This is the unsettling promise of mirror life, a cutting-edge area of synthetic
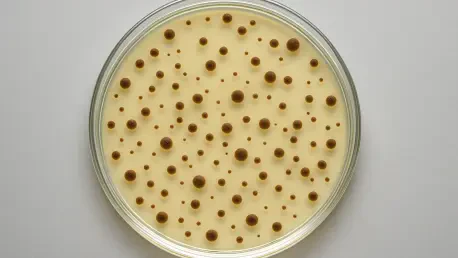
The Colony Counters Market is witnessing an unprecedented surge, fueled by groundbreaking technological advancements and a pressing need for precision in microbial analysis across various industries. A recent comprehensive report underscores how these specialized devices, designed to count and
The fight against cancer is entering a bold new era, where the intricacies of tumor biology are reshaping the very foundation of clinical trials and therapeutic development, moving beyond outdated methods to address the real challenges of treatment resistance. For too long, cancer treatments have
Small cell lung cancer (SCLC) continues to pose a significant challenge in oncology, with its aggressive progression and limited treatment avenues leaving patients and clinicians grappling for solutions. Representing roughly 10-12% of all lung cancer diagnoses worldwide, SCLC is notorious for rapid
Synthetic biology stands at the forefront of a transformative wave in the industrial enzymes market, promising to reshape how industries approach efficiency, sustainability, and innovation over the next decade. This cutting-edge field merges genetic engineering, DNA synthesis, and protein design to

In a landscape where technology is reshaping the delivery of healthcare to veterans, a significant development has emerged with GovCIO's acquisition of SoldierPoint Digital Health, previously the telehealth arm of Iron Bow Technologies. This strategic move signals a potential transformation in how

As we dive into the forefront of brain health research, I'm thrilled to speak with Ivan Kairatov, a biopharma expert with extensive experience in technology and innovation within the industry. With a strong background in research and development, Ivan brings unique insights into the newly
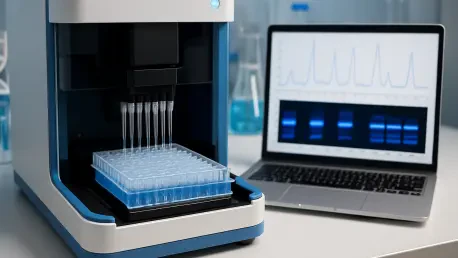
In the fast-paced world of life sciences, where precision and speed often determine the success of groundbreaking research, laboratory automation has emerged as a game-changer, particularly in the realm of nucleic acid analysis. Imagine a scenario where researchers spend less time on repetitive
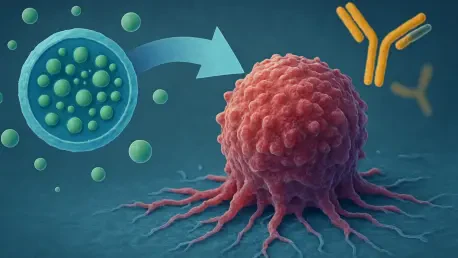
In the relentless battle against cancer, a disease that continues to challenge medical science with its complexity and resilience, a promising new direction has emerged through the targeting of platelet-activating factor (PAF), a potent phospholipid mediator intricately involved in tumor

How can a tiny tool transform the high-stakes world of laboratory automation, where precision dictates success and every second matters? Picture a bustling biotech lab, with samples piling up and researchers racing against time to deliver results. In such environments, even the smallest error can
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy