

In a landscape where technology is reshaping the delivery of healthcare to veterans, a significant development has emerged with GovCIO's acquisition of SoldierPoint Digital Health, previously the telehealth arm of Iron Bow Technologies. This strategic move signals a potential transformation in how

As we dive into the forefront of brain health research, I'm thrilled to speak with Ivan Kairatov, a biopharma expert with extensive experience in technology and innovation within the industry. With a strong background in research and development, Ivan brings unique insights into the newly
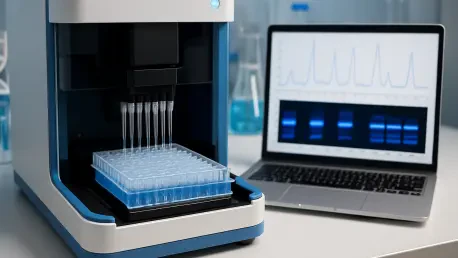
In the fast-paced world of life sciences, where precision and speed often determine the success of groundbreaking research, laboratory automation has emerged as a game-changer, particularly in the realm of nucleic acid analysis. Imagine a scenario where researchers spend less time on repetitive
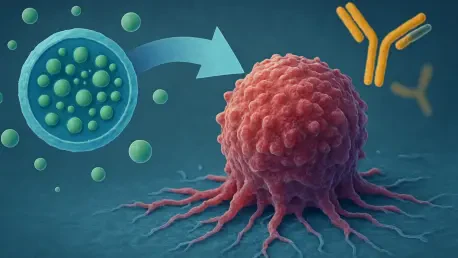
In the relentless battle against cancer, a disease that continues to challenge medical science with its complexity and resilience, a promising new direction has emerged through the targeting of platelet-activating factor (PAF), a potent phospholipid mediator intricately involved in tumor

How can a tiny tool transform the high-stakes world of laboratory automation, where precision dictates success and every second matters? Picture a bustling biotech lab, with samples piling up and researchers racing against time to deliver results. In such environments, even the smallest error can

In an era where smartphone cameras have become essential tools for capturing life's moments, the quest for superior imaging quality continues to drive innovation across the tech landscape, and a groundbreaking collaboration between TECNO, a leading global smartphone brand, and DXOMARK, a trusted
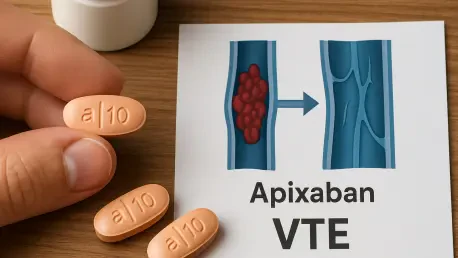
Imagine a patient recovering from a provoked venous thromboembolism (VTE), such as deep vein thrombosis (DVT) triggered by surgery, only to face an ongoing risk of recurrence due to chronic conditions like obesity or inflammatory disorders. With recurrence rates posing a significant threat to

In a world where technological advancements hinge on the tiniest of materials, researchers at North Carolina State University have introduced a game-changing innovation that promises to reshape the landscape of materials science. Named Rainbow, this multi-robot, self-driving laboratory leverages
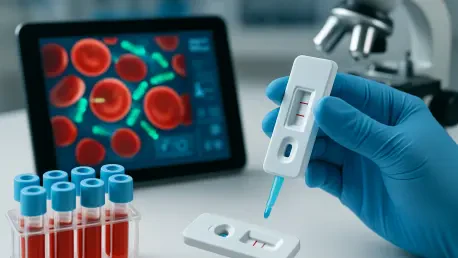
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, and a deep understanding of technological innovation in the medical field. Today, we're diving into a groundbreaking advancement in sepsis detection that promises to

In a troubling development for healthcare in Worcester, Massachusetts, Saint Vincent Hospital, operated by a prominent for-profit hospital system based in Texas, continues to grapple with severe patient safety issues that have sparked widespread concern among staff and regulators alike. Nurses and
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy