
I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research and development, and a deep understanding of cutting-edge technologies in the industry. Today, we’re diving into a groundbreaking advancement in mapping RNA-protein interactions—a

In a quiet corner of Devon, UK, Jason Yeo, a 54-year-old father of two and a traveling sales manager, faced a challenge he never saw coming—a prostate cancer diagnosis in April 2024 that could have easily slipped under the radar. With no prior health issues or noticeable symptoms, his story serves
Today, we’re thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with extensive experience in research, development, and technological innovation within the healthcare industry. Ivan brings a wealth of knowledge about how cutting-edge advancements, like the recent diagnostic

In the battle against infectious diseases like tuberculosis (TB) and malaria, which claim nearly 2 million lives each year in low- and middle-income countries (LMICs), clinical trials serve as a critical frontline for developing life-saving treatments and preventive measures. These regions, often

In an era where personalized healthcare is reshaping the landscape of medical treatment, the WIN Symposium stands as a beacon of innovation, and this year, it is set to make a profound impact under the leadership of Dr. Wafik S. El-Deiry, MD, PhD, FACP, Editor-in-Chief of Oncotarget . Scheduled for
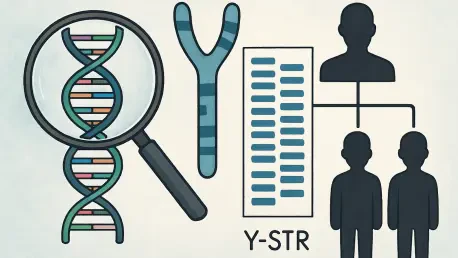
In the intricate world of forensic genetics, Y-chromosome short tandem repeats (Y-STRs) have long served as cornerstone markers for identifying male individuals, tracing paternal lineages, and untangling complex DNA mixtures in criminal investigations. These genetic signposts, passed down

In the fast-evolving world of pharmaceuticals, the first half of this year has delivered a stunning reshuffle in the hierarchy of blockbuster drugs, with Novo Nordisk’s Ozempic and Eli Lilly’s Mounjaro vaulting into the top three revenue earners. This dramatic rise, fueled by an insatiable demand

In the chaotic pulse of an emergency room, where every second can tip the balance between life and death, a staggering reality unfolds: millions of patients flood these high-stakes environments yearly, often outnumbering the staff available to save them. Picture a nurse, sweat beading on their
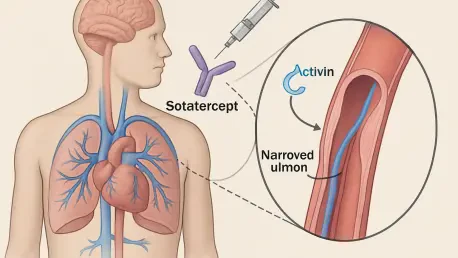
Pulmonary arterial hypertension (PAH), a life-threatening condition characterized by dangerously high blood pressure in the lung arteries, has long posed significant challenges for patients and clinicians alike due to its progressive nature and limited treatment options. Enter sotatercept, a

I'm thrilled to sit down with Ivan Kairatov, a renowned expert in environmental health and vision science, whose groundbreaking work has shed light on the critical link between air quality and children's eyesight. With a deep background in biopharma and a passion for leveraging technology to
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy