
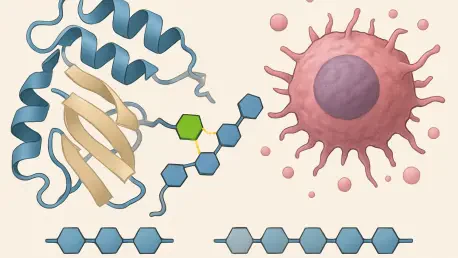
In the intricate world of cellular biology, a silent player often dictates the fate of health and disease: enzymes that modify proteins on a molecular level, shaping their function in profound ways. One such enzyme, N-acetylglucosaminyltransferase-V, commonly referred to as GnT-V or MGAT5, has

I'm thrilled to sit down with Ivan Kairatov, a renowned biopharma expert with a wealth of experience in research and development, particularly in the intersection of technology and innovation in the industry. Today, we’re diving into a fascinating area of study: the protective effects of maternal

Autism Spectrum Disorder (ASD) has traditionally been understood as a condition manifesting in early childhood, yet a pioneering study from the University of Cambridge is turning this perspective on its head with revelations about the genetic and developmental differences tied to the timing of
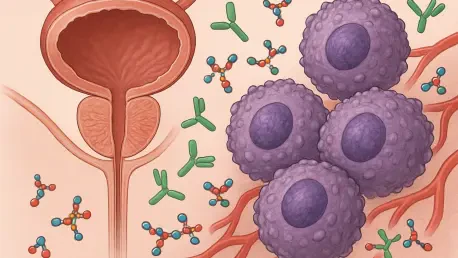
In a significant leap forward for prostate cancer research, a recent study published in Nature Genetics on November 5 has spotlighted PTGES3 (Prostaglandin E Synthase 3) as a pivotal regulator of the androgen receptor (AR), a protein deeply implicated in the disease’s progression. Conducted by
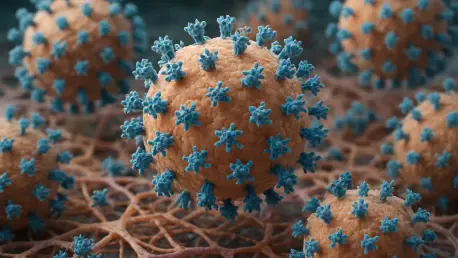
In the realm of virology, understanding how viruses interact with host cells is paramount to developing effective treatments and preventive measures, particularly for respiratory pathogens like the influenza A virus, which poses significant public health challenges. Traditional two-dimensional (2D)

In a world where obesity and type 2 diabetes affect millions, GLP-1 receptor agonists have emerged as transformative treatments, offering hope for better health outcomes. Yet, amidst their widespread adoption, a pressing concern lingers: could these powerful drugs, designed to regulate blood sugar

What if a simple combination of daily choices and modern medicine could halve the risk of heart attacks for millions living with Type 2 diabetes? Across the United States, where this condition affects over 30 million people, cardiovascular disease remains the leading cause of death for those
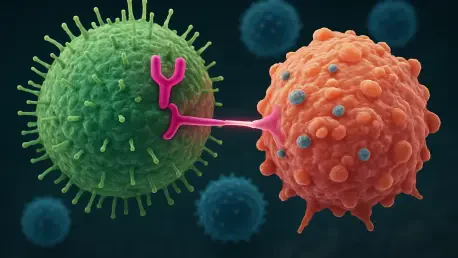
In the rapidly advancing field of cancer treatment, chimeric antigen receptor natural killer (CAR-NK) cell therapies stand out as a groundbreaking approach, offering scalable, off-the-shelf solutions that could transform patient care. These therapies, often developed from induced pluripotent stem

In the dynamic realm of scientific research, laboratories face an urgent need to adapt to a rapidly digitizing world where efficiency and precision are paramount, and a staggering amount of time is lost to navigating disconnected systems. Manually transferring data and wrestling with outdated

Imagine a world where a deadly brain cancer diagnosis in a child no longer carries the grim prognosis it does today, where a non-invasive technology could breach the brain’s natural defenses just long enough to deliver life-saving drugs directly to the tumor. This vision is becoming a tangible
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy