

The expansion of healthcare technology witnessed a significant milestone with the approval of Xenoview, a hyperpolarized contrast agent designed to enhance lung imaging for younger patients. The U.S. Food and Drug Administration (FDA) has provided the green light for this innovative approach,

In a groundbreaking leap towards enhancing the privacy of healthcare data, Barts Health NHS Trust has implemented an innovative imaging de-identification tool that streamlines previously cumbersome processes and safeguards patient information. Using Sectra’s 'anonymize and export' feature,

In an era defined by environmental challenges and a greater emphasis on corporate responsibility, the biotech industry is at a crossroads where sustainability is not just a choice but an imperative. With an increasing number of companies committing to environmental, social, and governance (ESG)

In an era where medical advancements are accelerating at an unprecedented pace, the collaboration between Danaher Corporation and AstraZeneca represents a pivotal moment for personalized healthcare. These two industry giants have joined forces to develop AI-powered diagnostics that promise to

The COVID-19 pandemic brought about a significant surge in the adoption of telemedicine services across various sectors, especially in home healthcare. With restrictions in place, telehealth became a vital tool for ensuring continuity of care while maintaining social distancing protocols. However,
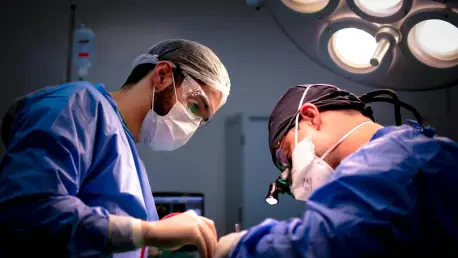
In the complex and highly competitive arena of the biotech and pharmaceutical industries, clinical operations serve as a vital linchpin, bridging the gap between scientific innovation and practical application. The success of clinical trials hinges not only on groundbreaking scientific discoveries

Artificial Intelligence (AI) has swiftly transitioned from speculative technology to a cornerstone of modern medical diagnostics, heralding a new era of healthcare innovation. As AI technologies integrate deeper into clinical settings, they are transforming the landscape of diagnostics by improving

In the rapidly evolving landscape of oncology, cancer vaccines have begun to emerge as pivotal players in the fight against solid tumors. As medical research continues to advance, innovative approaches are being explored to counteract the mechanisms by which cancer cells evade immune system

The medical community continuously seeks ways to improve patient care and outcomes, and platelet analysis is becoming a focal point of innovation. Platelets, critical components in the human circulatory system, are involved in the formation of blood clots, a process crucial for preventing excessive
The cell and gene therapy (CGT) sector stands on the precipice of revolutionary change, reminiscent of the fast food industry’s notable standardization achievements. This provocative comparison emerged during a recent discussion at BPI Europe in Hamburg, where industry leaders delved into the
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy