
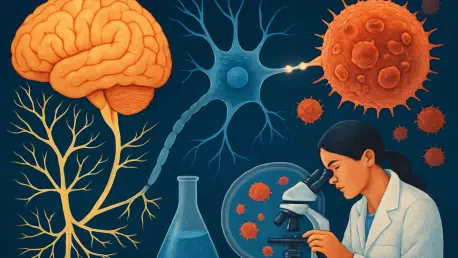
What happens when the body's own communication network turns against it, fueling a silent enemy within, and how can this discovery transform the fight against cancer? Gastrointestinal cancers, which claim millions of lives each year, have revealed a startling accomplice: the nervous system.

Diving into the gritty world of hardcore and metal, I’m thrilled to sit down with Billy Graziadei, the powerhouse frontman of Biohazard, a band that’s been a cornerstone of New York hardcore for decades. With their latest track “Death of Me” already making waves and a new album, Divided We Fall ,

The landscape of Medicaid in the United States stands at a critical juncture, with a staggering 18.5 million enrollees across 42 states and Washington, D.C., grappling with stringent work requirements that mandate at least 80 hours per month of work, study, volunteering, or job training to maintain

Imagine a world where the shadow of incurable diseases no longer looms over millions, where conditions like Alzheimer’s, heart failure, and spinal cord injuries are not just managed with temporary relief but treated at their very core through therapies that heal at the cellular level. Stem cell

In the fast-evolving landscape of biopharmaceutical production, ensuring the consistency of cell culture media stands as a cornerstone for achieving reliable cell growth and high-quality therapeutic products, especially given the complexity of these media formulations. With hundreds of components
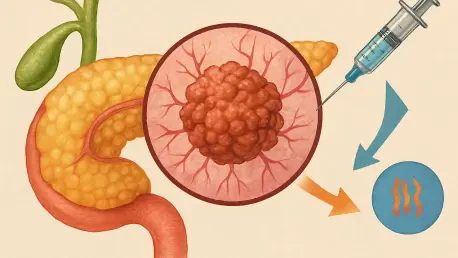
Pancreatic cancer stands as one of the most formidable adversaries in the realm of oncology, with survival rates often tragically limited to mere months after diagnosis, highlighting the urgent need for innovative solutions. The relentless nature of this disease, coupled with its resistance to
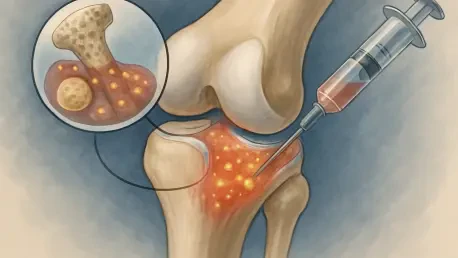
Could a single injection harness the body’s own healing power to mend a torn ligament or damaged cartilage, slashing recovery time for athletes and active individuals? This isn’t a distant dream but a reality unfolding through Bone Marrow Aspirate Concentrate (BMAC) therapy, a groundbreaking

The healthcare sector is on the cusp of a groundbreaking transformation, driven by the immense potential of artificial intelligence (AI) to revolutionize patient care, optimize operational workflows, and confront the mounting challenges of an aging demographic with increasingly intricate medical

Major policy changes are colliding with the complex economics of drug development. For B2B biopharma leaders, the Inflation Reduction Act fundamentally alters how companies approach pricing, launch, and life-cycle management; Medicare can now negotiate prices, cap price increases to inflation, and
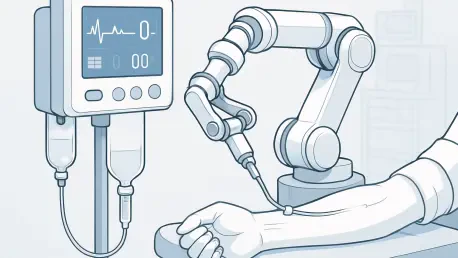
The medical device manufacturing sector is undergoing a seismic shift, with automation emerging as the cornerstone of innovation and efficiency as the industry looks toward 2035, driven by the urgent need to meet rising global healthcare demands. Projections from recent market analyses indicate
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy