The American Hospital Association (AHA) has put forth a series of recommendations aimed at reforming policies to mitigate health insurers' growing dominance in the market, focusing on recent developments that exacerbate competition challenges. The AHA has submitted these recommendations to the
In a significant advancement for Alzheimer's disease diagnosis, the US Food and Drug Administration (FDA) has approved a new and groundbreaking diagnostic tool, the Lumipulse G pTau217/β-Amyloid 1-42 Plasma Ratio blood test. Developed by Fujirebio Diagnostics, this test offers a less invasive

The legislative process in Nebraska surrounding medical cannabis has been fraught with complexities, illustrating a profound disconnect between public opinion and political action. Despite overwhelming voter approval in recent elections, Legislative Bill 677, aimed at providing a regulatory

The recent inauguration of the NIHR Bradford and West Yorkshire Commercial Research Delivery Centre (CRDC) marks a significant milestone in enhancing healthcare access in West Yorkshire. Established by the Bradford Teaching Hospitals NHS Foundation Trust, this clinical research hub is part of an
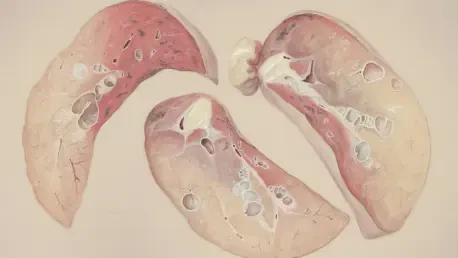
In recent months, the world of oncology has witnessed a remarkable milestone, bringing a glimmer of hope to thousands of patients battling advanced lung cancer. With the US Food and Drug Administration (FDA) speeding up approval for a groundbreaking drug, the focus has intensified on a specific

In a bold move to revolutionize the landscape of healthcare administration, the U.S. Department of Health and Human Services (HHS) has introduced the innovative "10-to-1" rule, signaling a significant shift in regulatory strategies. The initiative targets the reduction of bureaucratic