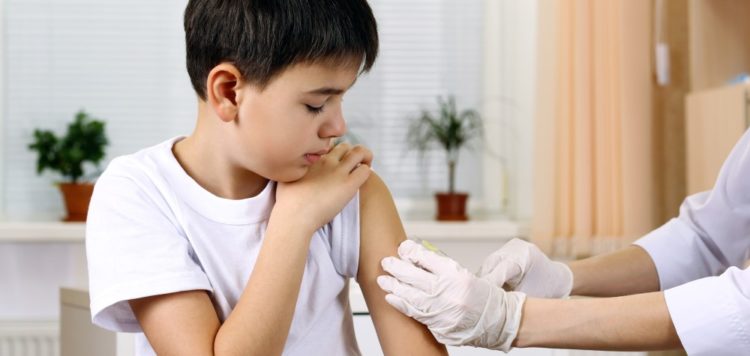
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted 21 to zero in favour of both vaccines in this age group, in light of rising rates of hospitalisations and deaths that have been seen in the very young during the Omicron wave of infections.
The panel said the emergency use authorisations for the shot should be amended to include children aged six months to five years for Moderna’s two-dose regimen, and six months to four years for Pfizer’s three-shot course.