There is no question that the recent COVID-19 pandemic has not only dramatically affected the lives of people around the world, but also shed light on the many shortcomings plaguing the healthcare and biopharmaceutical industries. The healthcare systems of several countries around the world found themselves brought to their knees by the crisis, while pharmaceutical companies in the US and abroad raced against time to develop, test, and deliver the much-needed vaccines and treatments. However, with time and the development of new vaccines and medicines, both industries were able to overcome the pandemic and help people regain their health.
The COVID-19 pandemic also demonstrated the importance of developing new drugs and making them widely available to those who need them. However, innovation is by no means an easy task, and it can become increasingly complicated in the biopharmaceutical industry. According to a study on the importance of new drug discovery companies, understanding the elements that drive drug innovation is critical to advancing healthcare and the future of companies that specialize in treatment discovery, research and development. That’s what makes the next five new drugs so interesting. They could transform healthcare for the better, not only for doctors and patients, but also for rival companies that hope to make similar discoveries.
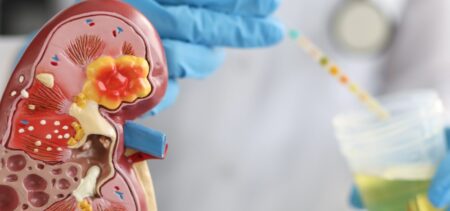
A New Drug Could Help Patients Suffering from Anemia Caused by Kidney Disease
According to BiopharmaDive, the Food and Drug Administration (FDA) is currently reviewing a new drug developed by GlaxoSmithKline (GSK). The new drug, called daprodustat, is considered to be a suitable treatment for anemia caused by kidney disease. Daprodustat was the third drug of its kind to be reviewed by the FDA in 2021. While the federal agency has already rejected two drugs similar to daprodustat and required new trials for both, GSK remains confident that its medicine has a better chance of being approved in the US. According to the same source, the pill is already available in Japan under the name Duvroq and is also currently under review in Europe.
A New Pill Could Bring Chronic Coughing Under Control
Gefapixant, a new oral drug, could soon prove useful in treating patients who suffer from unexplained chronic cough, according to The Guardian. The news website mentions that Prof. Surinder Birring has recently led a global trial that found the drug was effective in reducing a patient’s coughing by up to 60%. According to Birring, the now experimental treatment could be the first in over 50 years to effectively treat the symptoms of this serious condition. Although scientists have not yet been able to understand what exactly triggers chronic cough, apart from some known conditions such as asthma, the disease can seriously affect patients’ lives.
An Eczema Treatment Could Also be Used for Treating Young Children
According to The Lancet, a drug called dupilumab, which has already been approved by the FDA for the treatment of older children and adults suffering from atopic dermatitis or eczema, is also safe and highly effective for infants and young children. The research indicates that dupilumab significantly improved eczema symptoms in children younger than 6 years when compared to placebo, and it was also “well tolerated and showed an acceptable safety profile, similar to results in older children and adults.” Parents and caregivers could soon benefit from using dupilumab to treat their infants and children suffering from atopic dermatitis.
A New Drug Could Slow Cognitive Decline Associated with Alzheimer’s Disease
Lecanemab could slow the cognitive decline associated with Alzheimer’s disease, according to Medical News Today. The results of a phase 3 clinical trial seem to indicate that this new drug can actually delay the rate of cognitive decline in patients suffering from early Alzheimer’s disease by 27% after 18 months of treatment. However, it may be worth mentioning that the leading Spanish publisher, El País, has recently released information showing that the two pharmaceutical companies behind the development of lecanemab have provided “unverified data” about the drug, “even though many patients don’t notice any improvement.”
A New Treatment Could Slow Down the Symptoms of ALS
According to NPR, a new treatment could slow down the symptoms of amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or Lou Gehrig’s disease. The FDA has recently approved Relyvrio for use in the US. The federal agency based this decision on a single, relatively small-scale study. Although some FDA advisors objected to the new drug on the grounds that it might not work, the federal agency ultimately decided to approve it. Considering that ALS is an incurable, progressive nervous system disease that gradually destroys nerve cells and leads to most patients dying within two to five years of receiving the diagnosis, this can only be considered good news.
Conclusion
Pharmaceutical companies and scientists around the world are still searching for treatments that could cure or alleviate the symptoms of serious conditions, such as Alzheimer’s disease and ALS. It remains to be seen if drugs like Relyvrio and lecanemab can pave the way for even greater discoveries. However, with drugs like daprodustat, gefapixant and dupilumab showing promising results in treating other conditions, the future looks bright for the healthcare and biopharmaceutical industries.