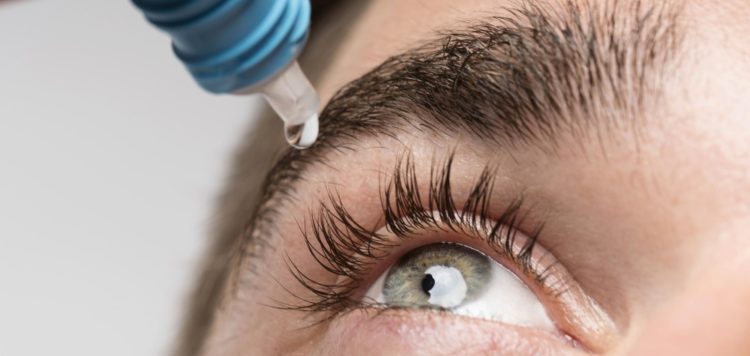
Visus Therapeutics Inc. (the “Company”), a clinical-stage pharmaceutical company in pursuit of developing the world’s first presbyopia-correcting eye drop with the potential to last a minimum of eight hours, today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Investigational New Drug Application (IND) to proceed with the clinical development program for BRIMOCHOL™, the company’s lead investigational asset. BRIMOCHOL, a proprietary combination of carbachol and brimonidine tartrate, is designed to be a once-daily eye drop to correct for the loss of near vision associated with presbyopia. Under this IND, Visus will initiate its planned Phase 2 clinical trial in the U.S. immediately.