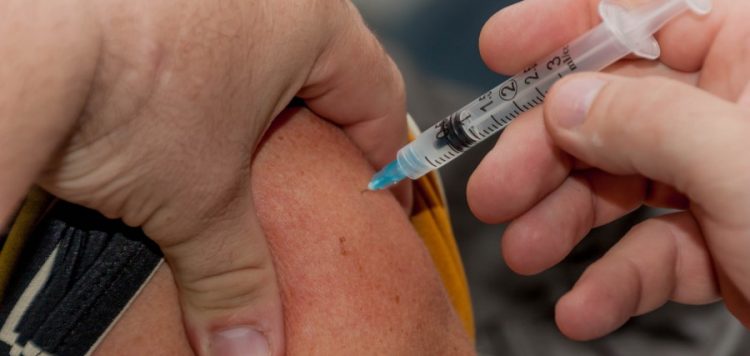
Much like a review of Pfizer and BioNTech’s vaccine last week, the FDA’s analysis of Moderna’s shot is similarly positive.
Results from a large trial testing the biotech’s vaccine in some 30,400 people showed two shots, spaced 28 days apart, were about 94% effective in preventing COVID-19, a finding affirmed by FDA scientists. The agency’s review was based primarily on an interim analysis of Moderna’s trial, which was based on 95 cases of COVID-19. Five were in vaccinated volunteers, while 90 occurred in participants who had received placebo.